文章来源:医脉通
2015年ASCO年会将于5月29日--6月2日在美国芝加哥召开。5月31日下午的肺癌免疫治疗专场,将公布Ⅰ期研究KEYNOTE-021队列D的研究结果。该研究结果表明,pembrolizumab加ipilimumab作为晚期非小细胞肺癌的二线治疗安全有效(摘要号8011)。医脉通对此进行了报道。
Pembrolizumab(pembro)是一种有效的抗PD-1单克隆抗体。Ipilimumab(IPI)是一种抗CTLA-4抗体,已经在晚期NSCLC中显示出有效性。对于黑色素瘤,抗PD-1和抗CTLA-4联合治疗已经显示出强大的功效和可控的毒性。我们将报告一项评估pembro + IPI治疗复发性NSCLC研究的中期结果。
相关报道:[2015 ASCO GI]KEYNOTE-012研究:Pembrolizumab用于胃癌安全有效
研究纳入了既往经≤2种治疗方案后复发的IIIB/IV期NSCLC患者。使他们接受 pembro + IPI 治疗,每三周一次,共4个周期,随后采用pembro维持治疗。基于nivolumab + IPI 治疗晚期NSCLC研究的新数据,pembro的剂量从10mg/kg 降到2mg/kg, IPI的剂量从3mg/kg降到1mg/kg。主要终点是安全和前3周给药剂量的剂量限制毒性(DLTs)发生率。调查者根据RECIST1.1评估缓解,每6周评估一次。
到2014年12月,入组17例患者:3位患者接受pembro 10mg/kg+IPI 3mg/kg、3位接受pembro 10mg/kg + IPI 1mg/kg,11位接受pembro 2mg/kg + IPI 1mg/kg。分析时,治疗的15例患者没有发生DLTs或剂量调整。10例患者发生药物相关的AEs(DRAEs)但没有人停止治疗或死亡。有2次3度 DRAEs,都是皮疹。2度DRAE是腹泻和呕吐(各2例)、寒战,咳嗽,食欲减退,体重下降,脱水,抑郁症,发音困难,乏力,肌痛,皮肤瘙痒和发热(各1例)。分析时,治疗 ≥ 6周时各剂量组的11位患者都可观察到缓解,其中包括1例CR(9%)和5例PRs(45%)(见表);所有的患者都获得了疾病控制。12例pts仍然在治疗(范围6+到26+周);3位患者由于PD停药。
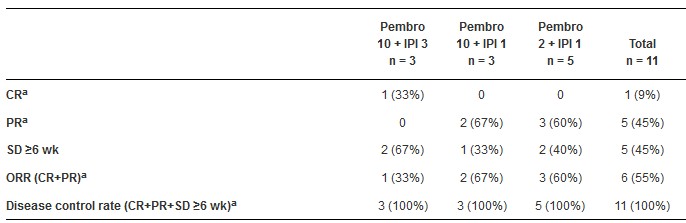
KEYNOTE-021 队列D的初始数据表明,pembro+ IPI对于复发性NSCLC患者,具有可接受的毒性作用和强大的抗肿瘤活性。使用较低剂量的pembro和IPI不会降低效果。临床试验信息:NCT02039674
会议专题》》》2015年ASCO年会专题报道
摘要原文(摘要号8011)
Background: Pembro is a potent anti–PD-1 monoclonal antibody. IPI, an anti–CTLA-4 antibody, has shown activity in advanced NSCLC. In melanoma, combined anti–PD-1 and anti–CTLA-4 treatment has shown robust efficacy and manageable toxicity. We report interim results from a phase 1 study evaluating pembro + IPI in patients (pts) with recurrent NSCLC.
Methods: Pts with stage IIIB/IV NSCLC that recurred after ≤ 2 prior regimens received pembro + IPI every 3 wk for 4 cycles followed by maintenance pembro. Based on emerging data from the nivolumab + IPI advanced NSCLC study, doses were reduced from 10 mg/kg to 2 mg/kg for pembro and from 3 mg/kg to 1 mg/kg for IPI). Primary end point was safety and incidence of dose-limiting toxicities (DLTs) in the first 3 wk of dosing. Response was assessed every 6 wk per RECIST 1.1 by investigator review.
Results: As of Dec 2014,17 pts were enrolled: 3 at pembro 10 mg/kg + IPI 3 mg/kg, 3 at pembro 10 mg/kg + IPI 1 mg/kg, and 11 at pembro 2 mg/kg + IPI 1 mg/kg. No DLTs or dose modifications were reported for the 15 pts treated at the time of analysis. 10 pts experienced drug-related AEs (DRAEs); none led to discontinuation or death. There were 2 gr 3 DRAEs, both rash. Gr 2 DRAEs were diarrhea and vomiting (n = 2 each) and chills, cough, decreased appetite, decreased weight, dehydration, depression, dysphonia, fatigue, myalgia, pruritus, and pyrexia (n = 1 each). Responses were seen in all dose groups among the 11 pts on treatment for ≥ 6 wk at the time of analysis, including 1 CR (9%) and 5 PRs (45%) (Table); all pts achieved disease control. 12 pts remain on treatment (range, 6 + to 26 + wk); 3 pts discontinued for PD.
Conclusions: Preliminary data from KEYNOTE-021 cohort D demonstrate an acceptable toxicity profile and robust antitumor activity for pembro + IPI in pts with recurrent NSCLC.The use of lower pembro and IPI doses did not appear to negatively impact efficacy. Clinical trial information: NCT02039674




